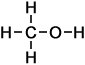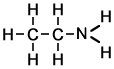How to draw Organic Molecules in 3D
Drawing Organic Molecules (in general)
There are several standard ways to represent the structures of organic molecules in different levels of detail.
These include:
- Molecular Formulae of Organic Molecules
- Structural Formulae of Organic Molecules:
- Fully Displayed Formulae
- Simpler Displayed Formulae
- Sketched 3D Formulae
- Modelled 3D Formulae
- Skeletal Formulae
For more about drawing organic molecules generally see: How to Draw Organic Molecules.
Why draw organic molecules in 3D ?
Molecules are 3-dimensional (3D) structures so it is always more accurate to describe them in 3D.
Representations of organic molecules in 2D, such as fully displayed formulae, are often used for speed and simplicity. While they show the atoms in the molecule and the connections between them, other important information may be omitted by representation in 2D only.
The benefits of drawing, some people prefer to say 'sketching molecules in 3D', include the possiblity of including information about:
- Bond Angles, and therefore the
- Shapes of molecules
and - Isomers, including stereoisomers.
Symbols used to draw organic molecules in 3D
i.e. to draw (or 'sketch') 3-D Structural Formulae
Symbols commonly used to represent the 3-dimensional (often called simply '3D') arrangements of atoms in organic molecules include:
For Comparison:
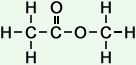
Note that in the case of 3D sketches of organic molecules, bonds represented by solid lines are understood to lie in the plane of the diagram (so, often 'in the plane of the paper', but in the plane of the screen if viewed electronically).
These are sometimes referred to a 'normal' bonds because of the simple lines used to represent them. So,
- 'Normal' lines (or 'normal bonds') are used to represent covalent bonds lying in the plane of the drawing surface, i.e. paper (textbook) or screen (if viewed electronically, e.g. online).
'dashed' and 'wedged' bonds are used to represent covalent bonds projecting out of this plane, either away from or towards the viewer. - By the way, these are referred to as the "bonds" they represent rather than as 'lines' (in the diagram) because the symbol for a 'wedged bond' is not really a line, but a slim triangle, so long and thin that it is referred to by its 'wedge' shape.
- Dashed bonds are used to represent bonds that project backward (behind the drawing plane).
- Wedged bonds are used to represent bonds that project outward or 'forward' (in front of the drawing plane).
Example of a simple sketched 3D Structural Formula
A simple example of a 3D Structural Formula is that for methane, whose molecular formula is CH4.
Compare the following two representations of methane (CH4):
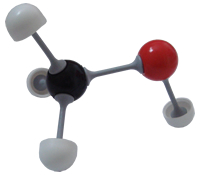
The sketched 3D structural formula of methane (on the left) may be understood even more clearly by comparison with the ('ball and stick') model of the 3D structure of methane (on the right).
Review the meanings of the dashed line and thin-wedge symbols given in the text above, in the context of their use in the sketched 3D structural formula of methane in the diagram.
Some further examples of how to draw organic molecules in 3D are included below, together with corresponding 'ball and stick' models for comparison.
Tips: If you have access to a suitable organic chemistry modelling kit (either in school / college, or you can buy your own) you may find it very useful for working-out how to draw organic molecules in 3D: First make a model of the correct structure, then define the plane of the diagram and hold the model in that orientation while you notice which of the bonds that do not lie in the plane of the diagram are coming (outwards) towards you, and which are pointing away from you.
More examples of how to draw organic molecules in 3D:
Methanol (CH3OH)
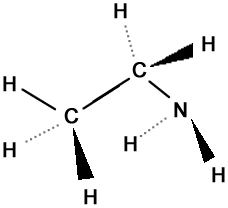
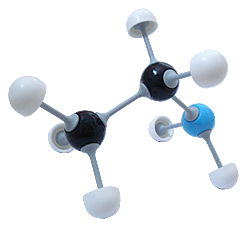
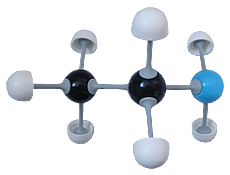
Ethylamine (C2H7N)
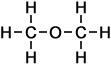
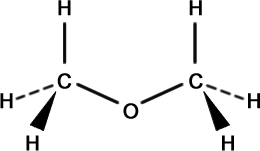
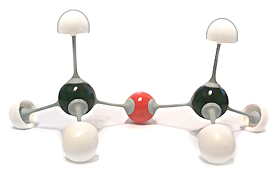
Methoxy Methane (C2H6O)
Molecule |
3D Sketch of molecule |
'Ball & Stick' 3D Model of molecule |
Methanol (CH3OH) |
||
|
|
|
|
Ethylamine (C2H7N) |
||
|
|
|
|
Methoxy Methane (C2H6O)
|
||
Notes about these 3D sketches of organic molecules:
Sometimes 3D sketches of organic molecules can look awkward and messy, espesically when drawn in haste e.g. during an explanation or discussion, or in an exam. That is often unavoidable so if you have to produce diagrams of this type do not be too concerned about their artistic appearance.
The important aspects of such diagrams are to show all the correct atoms linked xssto the correct other atoms (as is also true for full displayed formulae) and to use correctly, representation of the three different styles indicating:
-
 bonds that lie in the plane of the diagram (paper, or screen),
bonds that lie in the plane of the diagram (paper, or screen), -
 bonds whose orientation in 3D space is towards the viewer (so the main carbon-chain or approx. 'centre' of the molecule would be further from the viewer than the atom or functional group attached to it by a bond extending towards the viewer)
bonds whose orientation in 3D space is towards the viewer (so the main carbon-chain or approx. 'centre' of the molecule would be further from the viewer than the atom or functional group attached to it by a bond extending towards the viewer)  bonds whose orientation in 3D space is away from the viewer (so the main carbon-chain or approx. 'centre' of the molecule would be closer to the viewer than the atom or functional group attached to it by a bond extending away from the viewer).
bonds whose orientation in 3D space is away from the viewer (so the main carbon-chain or approx. 'centre' of the molecule would be closer to the viewer than the atom or functional group attached to it by a bond extending away from the viewer).
The priority when sketching 3D molecules using this system is to convey more information than is included in the corresponding fully displayed formula by indicating approximate bond angles / orientations using the 3 styles of representation of bonds in 3D space.
When to draw organic molecules in 3D
Use 3D representations of the type illustrated above when you are asked to draw (or sketch) a molecule in 3D, or when this is necessary in order to answer a question, e.g. to explain the difference between optical isomers.
Although it is useful to be able to draw 3D molecular structures of organic compounds, and some examination courses require understanding of this and may require candidates to demonstrate it in exams, diagrams of this type can quickly become cumbersome and very difficult to draw clearly. For this reason you are unlikely to have to draw 3D molecular structures of very complicated molecules as part of a course in school-level chemistry.
A common use of this type of representation of molecular structures is to explain isomerism, and optical isomerism in particular. It is also important to be able to understand representations such as the 'Sketched' 3-D Structural Formula of Methane (above) when they are used in textbooks, reports, papers and other documents.
In the context of high school level (incl. UK GCSE and A-Level) chemistry, students often need to be able to draw the Fully Displayed Formulae and / or the Simplified Displayed Formulae of a wide range of organic molecules.
In most cases it is only necessary to be able to draw the 3D Structural Formulae of a smaller number of specific organic molecules, especially those that exhibit optical isomerism. However, it is really useful to be familiar with the 3D structures and hence the real shapes of organic molecules generally.
See also our main page about how to draw organic molecules.