
Naming Ethers
Definition of Ethers:
Ethers are organic chemical compounds whose structure has the general form:
where the symbols R1 and R2 represent organic radicals, usually carbon chains.
Names of Ethers in General
Ethers are named according to the standard system of naming organic compunds. As is also true for other types of organic compounds / molecules, there are also some non-standard names for ethers in common usage. Some of the alternative names used for the simple ethers shown in the tables of examples on this page are listed in the column under the header 'synonyms'.
A standard system for naming ethers follows the system alkoxy- as in the following example:
This shows that ethers consist of two parts (often carbon chains), labelled as R1 and R2 at the top of this page.
When working-out the name of an ether given its molecular structure, the first steps are:
- Recognise that the molecule is an ether because it has the general form:

- Identify the parts labelled
R1 and R2.
To do this recall the standard system of labelling carbon chains as used for alkanes.
Note that R1 and R2 might be linear carbon chains (which are simpler to name) or they might be branched, or might even have other functional groups, e.g. halogens, see haloalkanes, attached as well. - According to the standard system for naming ethers, the shorter of the two chains R1 and R2 becomes the first part of the name with the '-ane' suffix that applies to the corresponding alkane changed to -oxy, and the name of the longer alkane chain forming the suffix of the name of the ether.
Hence, CH3OCH2CH3 is methoxyethane (not ethoxymethane).
However, as can be seen from the lists of synoyms in the extreme right column of the following tables, organic molecules are not always known only by their correct / standard names. An understanding of the general system for naming ethers described here is useful to enable recognition of e.g. 'ethoxymethane' as having the molecular structure of methoxyethane, even if the compound / molecule has not been referred to using the name expected according to this standard system.
Also ...
Note that the system 'alkoxy- alkane' is not the only method used for naming ethers.
Another system for naming ethers works by citing the names of the groups R1 and R2 in alphabetical order, followed by the class name 'ether'. So, for example 'methoxy ethane' = 'ethyl methyl ether'
In general, the larger and more complicated the organic compound, the more different naming systems there are and the more variations can be found.
Simple diagrams of the molecular structures of ethers are shown below. Ease of naming ethers and drawing the molecular structures of ethers comes with experience. It helps to consider a series of simple examples in order to recognise patterns in the naming of ethers that can then be applied to similar as well as larger and more complicated examples.
Names and Structures of simple Linear Ethers
in which R1 = CH3, which is known as a "-methyl group"
The homologous series of linear ethers that include a -methyl group attached (via a single covalent bond) to the oxygen atom in the middle of the ether molecule. This is easier to understand in conjunction with viewing the molecular structures in the table:
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
1 |
methoxy methane |
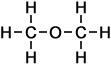 |
|
2 |
methoxy ethane |
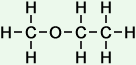 |
|
3 |
methoxy propane |
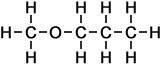 |
|
4 |
methoxy butane |
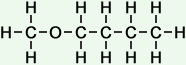 |
|
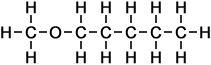
5 |
methoxy pentane |
 |
|
- methoxy methane (C2H6O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methoxy ethane
(C3H8O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methoxy propane
(C4H10O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methoxy butane
(C5H12O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methoxy pentane
(C6H14O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Names and Structures of simple Linear Ethers
in which R1 = CH2CH3, which is known as an "-ethyl group"
The homologous series of linear ethers that include an -ethyl group attached (via a single covalent bond) to the oxygen atom in the middle of the ether molecule. This is easier to understand in conjunction with viewing the molecular structures in the table:
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
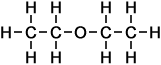
2 |
ethoxy ethane |
 |
|
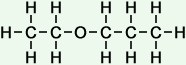
3 |
ethoxy propane |
 |
|
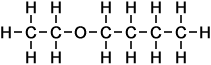
4 |
ethoxy butane |
 |
|
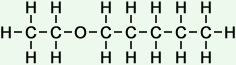
5 |
ethoxy pentane |
 |
|
- ethoxy ethane
(C4H10O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethoxy propane
(C5H12O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethoxy butane
(C6H14O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethoxy pentane
(C7H16O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Names and Structures of simple Linear Ethers
in which R1 = CH2CH2CH3, which is known as an "-propyl group"
The homologous series of linear ethers that include a -propyl group attached (via a single covalent bond) to the oxygen atom in the middle of the ether molecule. This is easier to understand in conjunction with viewing the molecular structures in the table:
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
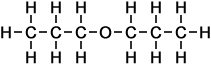
3 |
propoxy propane |
 |
|
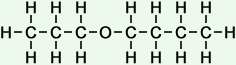
4 |
propoxy butane |
 |
|
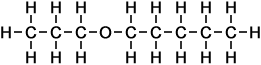
5 |
propoxy pentane |
 |
|
- propoxy propane
(C6H14O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propoxy butane
(C7H16O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propoxy pentane
(C8H18O)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
See also the related page about functional groups in organic molecules.









