
Naming Nitriles
Definition of Nitriles:
Nitriles are organic chemical compounds that include the functional group -C≡N
That is, nitriles are a class or category of chemical compounds that include a nitrogen atom connected to a carbon atom by a triple covalent bond.
Nitrile molecules can vary in size up to very long molecules most of which consist of carbon atoms attached to each other and also to hydrogen atoms.
Names of Nitriles in General
Many chemicals and categories of chemicals have been known by different (general, and specific) names in the past.
Nitriles used to be known as cyanides.
This page indicates the modern names, chemical structures, and some of the previous and less common names of some simple nitriles. It is useful to know and recognise previous and unusual names of nitriles but when naming nitriles in coursework and exam questions the modern names of nitriles are generally expected.
The smallest chemical whose molecular structure includes a nitrogen atom connected to a carbon atom by a triple covalent bond is hydrogen cyanide (HCN), whose structure may be drawn as simply: H-C≡N . However, because HCN does not include a carbon chain (of at least two carbon atoms linked together) it does not always count as a true 'organic molecule'. Therefore lists of linear nitriles usually start with ethanenitrile, as below.
Names and Structures of simple Linear Nitriles
The homologous series of simple linear nitriles with the -nitrile group (i.e. a single nitrogen atom attached to a carbon atom by a triple covalent bond) attached to the first (=last) carbon atom is shown below for carbon chain lengths of up to 10 carbon atoms:
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Previous / Other Names |
|
2 |
ethanenitrile |
 |
|
3 |
propanenitrile |
 |
|
4 |
butanenitrile |
 |
|
5 |
pentanenitrile |
 |
|
6 |
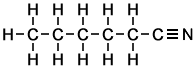
hexanenitrile |
 |
|
7 |
heptanenitrile |
 |
|
8 |
octanenitrile |
 |
|
9 |
nonanenitrile |
 |
|
10 |
decanenitrile |
 |
|
- Ethanenitrile ( 2 carbon atoms in chain )
Simple Formula:CH3CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- Acetonitrile
- Cyanomethane
- Methyl cyanide
- Ethanenitrile
- Ethyl nitrile
- Methanecarbonitrile
- Acetonitril
- Ethanonitrile
- Methylkyanid
- Ethane nitrile
- Propanenitrile ( 3 carbon atoms in chain )
Simple Formula:CH3CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- n-Propanenitrile
- Ethyl cyanide
- Propionitrile
- Propiononitrile
- Propylnitrile
- Cyanoethane
- Hydrocyanic ether
- Propionic nitrile
- Ethylkyanid
- Propannitril
- Butanenitrile ( 4 carbon atoms in chain )
Simple Formula:CH3CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- n-Butanenitrile
- Butyronitrile
- n-Butyronitrile
- Propyl cyanide
- n-Propyl cyanide
- Butyrylonitrile
- 1-Cyanopropane
- Butane nitrile
- Propylkyanid
- Butyric acid nitrile
- Pentanenitrile ( 5 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- n-Pentanenitrile
- Butyl cyanide
- 1-Butyl cyanide
- Valeronitrile
- n-Valeronitrile
- 1-Cyanobutane
- Hexanenitrile ( 6 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- Hexanonitrile
- Capronitrile
- n-Capronitrile
- Tricapronile
- Pentyl cyanide
- 1-Cyanopentane
- Amyl Cyanide
- n-Amyl cyanide
- Heptanenitrile ( 7 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- n-Heptanenitrile
- Heptanonitrile
- Hexyl cyanide
- Enanthonitrile
- 1-Cyanohexane
- Heptane nitrile
- Heptane-1-nitrile
- Octanenitrile ( 8 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- Heptyl cyanide
- Octanonitrile
- Caprylonitrile
- Caprylnitrile
- Arneel 8
- 1-Cyanoheptane
- Nonanenitrile ( 9 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CH2CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- Nonanitrile
- Nonanonitrile
- n-Octyl cyanide
- n-Nonanonitrile
- n-Octylcyanide
- Octyl cyanide
- 1-Cyanooctane
- n-Nonanenitrile
- Decanenitrile ( 10 carbon atoms in chain )
Simple Formula:CH3CH2CH2CH2CH2CH2CH2CH2CH2CNSimple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- n-Decanenitrile
- Nonyl cyanide
- Caprinitrile
- Decanonitrile
- 1-Cyanononane
See also the related page about functional groups in organic molecules, which includes the nitrile group among others.









