Naming Alkanes
Definition of Alkanes:
Alkanes are organic compounds that consist only of the elements carbon (C) and hydrogen (H) linked by single bonds.
Each carbon atom forms 4 bonds (either C-H or C-C bonds). Each hydrogen atom is connected to a single carbon atom, by a H-C bond.
Alkanes are hydrocarbons because they consist only of carbon and hydrogen atoms.
They combine in proportions according to the general formula for alkanes:
CnH2n+2
where the letter n represents the number of carbon atoms in each molecule of the compound.
The three main types of alkanes are linear alkanes, branched alkanes and cyclic alkanes (also called cycloalkanes). Linear alkanes are the simplest.
Linear Alkanes:
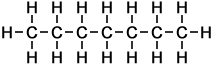
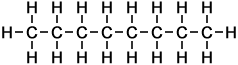
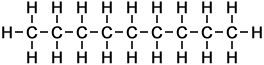
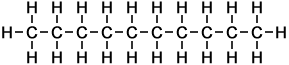
The first ten members of the homologous series of linear alkanes are shown below.
Name of Alkane |
No. C atoms |
Simple Structure |
Also known as |
No. of isomers |
|
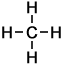
Methane |
1 |
C H4 |
|
|
1 |
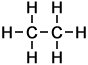
Ethane |
2 |
C2H6 |
|
|
1 |
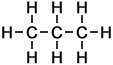
Propane |
3 |
C3H8 |
|
|
1 |
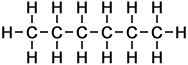
n-Butane |
4 |
C4H10 |
|
|
2 |
n-Pentane |
5 |
C5H12 |
|
|
3 |
n-Hexane |
6 |
C6H14 |
|
|
5 |
n-Heptane |
7 |
C7H16 |
|
|
9 |
n-Octane |
8 |
C8H18 |
|
|
18 |
n-Nonane |
9 |
C9H20 |
|
|
35 |
n-Decane |
10 |
C10H22 |
|
|
75 |
- Methane (CH4)
Number of carbon atoms: 1Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- natural gas
- marsh gas
- methyl hydride
Number of isomers: 1 - Ethane (C2H6)
Number of carbon atoms: 2Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- dimethyl
- methyl methane
- ethyl hydride
Number of isomers: 1 - Propane (C3H8)
Number of carbon atoms: 3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- dimethyl methane
- propyl hydride
Number of isomers: 1 - n-Butane (C4H10)
Number of carbon atoms: 4Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- methylethyl methane
- butyl hydride
Number of isomers: 2 - n-Pentane (C5H12)
Number of carbon atoms: 5Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- amyl hydride
- Skellysolve A
Number of isomers: 3
- n-Hexane (C6H14)
Number of carbon atoms: 6Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- dipropyl
- Gettysolve-B
- hexyl hydride
- Skellysolve B
Number of isomers: 5 - n-Heptane (C7H16)
Number of carbon atoms: 7Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- dipropyl methane
- Gettysolve-C
- heptyl hydride
- Skellysolve C
Number of isomers: 9 - n-Octane (C8H18)
Number of carbon atoms: 8Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- dibutyl
- octyl hydride
Number of isomers: 18 - n-Nonane (C9H20)
Number of carbon atoms: 9Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- nonyl hydride
- Shellsol 140
Number of isomers: 35 - n-Decane (C10H22)
Number of carbon atoms: 10Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:- decyl hydride
Number of isomers: 75
In the case of the names of alkanes beginning with n- , the n- part is included to specify the linear (as opposed to a branched or cyclic) form of that particular alkane. In some cases the atoms are arranged in different ways, hence the alkane can exist in the forms of several different structural isomers. Some examples of structural isomers are shown below.
Branched Alkanes
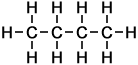
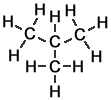
Alkane molecules that include more than 3 carbon atoms can be arranged in more than one way. The simplest example is butane, which can take either of two different forms called structural isomers:
The 2 Structural Isomers of Butane
Butane |
Methylpropane |
|
 |
 |
|
n-butane |
isobutane |
|
Another way to draw methylpropane that is less accurate, but perhaps easier to see initially, is :
The diagram on the right can be considered as the result of exchanging the carbon-group on the right-hand-side of the butane structure shown below, with the hydrogen atom indicated. However, the diagram on the (top) right is a less accurate representation of the actual molecule because the angles between the atoms in real 3-dimensional space are more similar to each other, as indicated in the molecular structure shown above.
Another way to draw methylpropane that is less accurate, but perhaps easier to see initially, is :
The diagram above can be considered as the result of exchanging the carbon-group on the right-hand-side of the n-butane structure with the hydrogen atom indicated. However, the diagram of the structure of methylpropane (above) is not an accurate representation of the actual molecule because the angles between the atoms in real 3-dimensional space are more similar to each other, as indicated in the molecular structure shown in the smaller diagram further above.
It is useful to be aware of structural isomers of organic molecules as the result of exchanging the positions of certain atoms and / or groups because this concept applies to many different types of organic molecules, not just to alkanes.
Another note about these diagrams is that, because they are very simple representations of the arrangements of atoms within molecules, the lengths and thicknesses of the lines representing chemical bonds do not generally signify anything about the bonds they represent. Although it is a good idea to draw these lines as consistently as possible, it is not unusual to need to vary the lengths slightly in order to fit everything in to the space available.
In molecular diagrams of organic molecules single lines represent single bonds, double lines represent double bonds, and triple lines represent triple bonds. The molecular diagrams on this page include only single bonds, which shows that alkanes are saturated molecules.
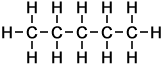
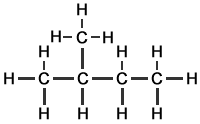
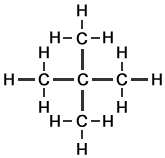
The 3 Structural Isomers of Pentane
Pentane (C5H12) has 5 carbon atoms per molecule. In organic chemistry generally, the larger the molecule, the greater the number of different ways in which the atoms can be arranged. The 3 structural isomers of pentane are shown.
Pentane |
Methylbutane |
Dimethylpropane |
|
 |
 |
 |
|
n-pentane |
isopentane |
neopentane |
As indicated in the table of linear alkanes further up this page, molecules of alkanes that have more carbon atoms per molecule can exist in many more different isomeric forms. Introductory courses, such as GCSE Chemistry, do not usually require detailed knowledge of all the isomers of the larger alkanes but it can be useful to work out and draw the molecular structures of some them to aid understanding and memory of the concept of structural isomerism.
More about alkanes generally (Revision Notes):
- Alkanes form a homologous series of organic compounds (meaning that all alkanes can be described by a single general chemical formula, specifically CnH2n+2 in the case of alkanes) in which the members of the series differ by a constant relative molecular mass of 14, which is the molecular mass of one carbon atom and two hydrogen atoms.
- Alkanes are saturated compounds, meaning that the atoms that form the molecules of alkane compounds are linked exclusively by single bonds.
- There is no limit to the number of carbon atoms that can be linked together in such a way as to form a linear alkane.
- The conditions that alkane molecules must satisfy are that they are:
- hydrocarbons (only contain atoms of carbon and hydrogen),
- saturated (only contain single bonds; so include no double- or triple- carbon bonds),
- Saturated oils and waxes are examples of larger alkanes in which the number of carbon atoms in the carbon chain, which is sometimes called a 'carbon backbone', is greater than 10.
- Alkanes are not very reactive and have little biological activity.
However, alkanes are sometimes described in terms of being a 'molecular tree' i.e. a basic structure, onto which more complex and biologically active atoms or functional groups of atoms can be attached, the simplest example being halogenoalkanes also known as haloalkanes.
See also: Boiling Points of Alkanes