
Naming Esters
Definition of Esters:
Esters are organic chemical compounds whose structure has the general form:

where the symbols R1 and R2 represent organic radicals.
- R1 and R2 are often carbon chains that can be either linear or branched and might also have other functional groups attached.
- R1 and R2 are not necessarily the same as each other, hence the 1 and 2 to distinguish them in the general definition of an ester. However, they can be the same, e.g. methyl ethanoate, ethyl propanoate, propyl butanoate and so on.
- R2 is not a hydrogen atom because if it were, the molecule would be a carboxylic acid rather than an ester.
The simplest esters are those where both R1 and R2 are "an alkane less the hydrogen atom at the end of the chain", and hence where the hydrogen atom at the end of the corresponding alkane is replaced by the carbon or oxygen atom to which that R (so, R1 or R2 in the diagram above) is attached.
Names of Esters in General
Esters are named according to the standard system of naming organic compunds. As for other types of organic compounds, there are also some non-standard names for esters. Some of the alternative names used for the simple esters shown in the tables of examples on this page are listed in the column under the heading Synonyms.
The standard system for naming esters uses the suffix -oate to indicate that a molecule is an ester.
The two organic radicals (which are often carbon chains) labelled
R1 and R2 in the diagram at the top of this page are also identified in the name of the compound.
This is shown using the example of ethyl propanoate:
The diagram above shows that esters consist of two parts (often carbon chains), as labelled R1 and R2 at the top of this page.
When working-out the name of an ester given its molecular structure, the first steps are:
- Recognise that the molecule is an ester because it has the general form:

- Identify the parts labelled
R1 and R2.
To do this recall the standard system of labelling carbon chains as used for alkanes.
Also take care to distinguish R1 and R2 by noticing which R the oxygen atom is attached to by a double bond.
Note that R1 and R2 may be linear charbon chains (which are simpler to name) or they may be branched, and they may even have other functional groups, e.g. halogens (see haloalkanes), attached as well.
Ease of naming esters and drawing the molecular structures of esters comes with experience. It helps to consider a series of simple examples in order to recognise patterns in the naming of esters that can then be applied to similar and larger and more complicated examples.
Names and Structures of simple Linear Esters
in which R2 = CH3, which is known as a "-methyl group"
The following table shows the first six members of the homologous series of linear esters that include a -methyl group attached (via a single covalent bond) to the oxygen atom in the chain of the ester molecule. This is easier to follow when viewing the molecular structures in the table:
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
1 |
methyl formate |
 |
|
2 |
methyl ethanoate |
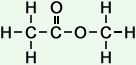 |
|
3 |
methyl propanoate |
 |
|
4 |
methyl butanoate |
 |
|
5 |
methyl pentanoate |
 |
|
6 |
methyl hexanoate |
 |
|
- methyl formate
(C2H4O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methyl ethanoate
(C3H6O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methyl propanoate
(C4H8O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methyl butanoate
(C5H10O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methyl pentanoate
(C6H12O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - methyl hexanoate
(C7H14O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Names and Structures of simple Linear Esters
in which R2 = CH2CH3, which is known as an "-ethyl group"
The following table shows the first six members of the homologous series of linear esters that include an -ethyl group attached (via a single covalent bond) to the oxygen atom in the chain of the ester molecule.
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
1 |
ethyl formate |
 |
|
2 |
ethyl ethanoate |
 |
|
3 |
ethyl propanoate |
 |
|
4 |
ethyl butanoate |
 |
|
5 |
ethyl pentanoate |
 |
|
6 |
ethyl hexanoate |
 |
|
- ethyl formate
(C3H6O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethyl ethanoate
(C4H8O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethyl propanoate
(C5H10O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethyl butanoate
(C6H12O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethyl pentanoate
(C7146O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - ethyl hexanoate
(C8H16O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Names and Structures of simple Linear Esters
in which R2 = CH2CH2CH3, which is known as an "-propyl group"
The following table shows the first six members of the homologous series of linear esters that include a -propyl group attached (via a single covalent bond) to the oxygen atom in the chain of the ester molecule.
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Synonyns: |
|
1 |
propyl formate |
 |
|
2 |
propyl ethanoate |
 |
|
3 |
propyl propanoate |
 |
|
4 |
propyl butanoate |
 |
|
5 |
propyl pentanoate |
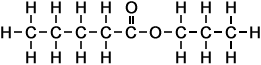 |
|
6 |
propyl hexanoate |
 |
|
- propyl formate
(C4H8O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propyl ethanoate
(C5H10O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propyl propanoate
(C6H12O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propyl butanoate
(C7H14O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propyl pentanoate
(C8H16O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - propyl hexanoate
(C9H18O2)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Why stop at six carbon atoms (before the -O- ) ? It doesn't. There are more linear esters.
See also the related page about functional groups in organic molecules.









