Homologous Series
Definition of a homologous series in organic chemistry:
A Homologous Series is a group of organic chemical compounds, usually listed in order of increasing size, that have a similar structure (and hence also similar properties) and whose structures differ only by the number of CH2 units in the main carbon chain.
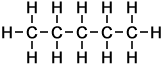
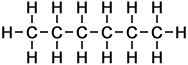
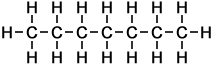
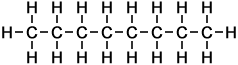
The simplest example of a homologous series in organic chemistry is that of alkanes (as taught for UK GCSE Chemistry, to ages approx 14-16 yrs). Alkanes consist of carbon and hydrogen atoms only, in proportions according to the general formula:
CnH2n+2
where the letter n represents the number of carbon atoms in each molecule of the compound. Hence the first 10 molecules in the homologous series of linear alkanes may be listed as follows (below, right):
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
Example of the Homologous Series of Alkanes, Structure: CnH2n+2
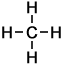
1 |
C H4 |
|
||
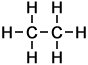
2 |
C2H6 |
|
||
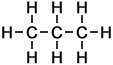
3 |
C3H8 |
|
||
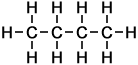
4 |
C4H10 |
|
||
5 |
C5H12 |
|
||
6 |
C6H14 |
|
||
7 |
C7H16 |
|
||
8 |
C8H18 |
|
||
9 |
C9H20 |
|
||
10 |
C10H22 |
|
||
As can be seen in the case of the example of the homologous series of alkanes (right), the basic molecular structure of all members of the series takes the same form. The difference between members of the homologous series depends on the value of n, which represents the number of carbon atoms in the chain.
The homologous series of alkanes is the simplest and probably most often cited example but there are many other homologous series of organic compounds.
Examples of some other important homologous series are below:
CnH2n+2 |
|
CnH2n |
|
CnH2n-2 |
|
CnH2n+1Br |
|
CnH2n+1OH |
|
CnH2nO |
|
CnH2nO2 |
|
CnH2n-1OCl |
|
CnH2n+1NH2 |
|
CnH2n-1ONH2 |
|
CnH2n-3N |
|
For more information, including diagrams of molecular structures and examples of each of the series listed above, visit the links on the name of each of the series.
Properties of Compounds within the same Homologous Series:
Two types of properties of substances (of all types, incl. elements, mixtures and compounds) are described within the subject of chemistry. They are the chemical properties of the substance and the physical properties of the substance.
1. Chemical Properties
Organic compounds that are part of the same homologous series generally have similar chemical properties as each other, due to the presence of the same functional group in the molecules of all compounds in the series.
Even though members of the same homologous series generally have similar chemical properties there may still be trends through the group (e.g. as reactivity and rates of reaction vary with parameters such as molecular weights).
2. Physical Properties
Physical properties of organic compounds that are part of the same homologous series follow trends through the series.
The physical properties of any particular member of a homologous series depends on its size, or (therefore) it's position within the homologous series.
A common example of trends within homologous series is that of the boiling points of the members of the series. See, for example, boiling points of alkanes.
As mentioned above, the trends in physical properties of compounds within a homologous series are primarily due to the progression of sizes and therefore weights of the molecules that form the homologous series. Using the example of the boiling points of alkanes, ethane having a higher boiling point than methane is explained by molecules of ethane (C2H6) having more Van der Waals forces (intermolecular forces) with neighbouring molecules than is true for methane (CH4) due to the greater number of atoms forming molecules of ethane, compared with methane.
More about Homologous Series:
- Successive members of a homologous series are called homologues.
- A homologation reaction is a chemical process which converts one member of a homologous series to the next member.
- We have occasionally seen 'homologous series' written as 'homologus series', which is a mis-spelling.