
Naming Acid Anhydrides
Definition of Acid Anhydrides:
Acid Anhydrides are organic chemical compounds whose structure has the general form:

where the symbols 'R1' and 'R2' usually represent carbon chains.
The simplest acid anhydrides to describe are those in which R1 = R2 = "an alkane less the hydrogen atom at the end of the chain", where the hydrogen atom at the end of the corresponding alkane is replaced by the carbon atom to which that "R" (so, R1 or R2 in the diagram above) is attached.
The above definition concerns only the molecular structure of acid anhydrides, but raises and does not answer two obvious questions:
- Why are acid anhydrides called 'acids' ?
and - Why are acid anhydrides called 'anhydride's ?
It is useful to be aware of the answers to these questions, which can be explained using an example.
Example of a simple Acid Anhydride:
Ethanoic Anhydride
The prefix 'eth-' usually indicates a chain of two carbon atoms (e.g. ethane, ethene, ethyne, ethanol, etc).
The simplest carboxylic acid based on a chain of two carbon atoms is ethanoic acid, whose structure may be represented as:
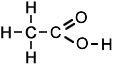 or more simply as:
or more simply as: ![]() .
.
By combining two molecules of a carboxylic acid in such a way as to remove or extract (from them) one molecule of water, an acid anhydride may be formed*.
*This statement should be understood theoretically rather than practically because acid anhydrides are not necessarily produced in this way.
For example, although it is possible to produce ethanoic anhydride by dehydrating ethanoic acid, it is usually made via a more efficient though less obvious process (probably beyond the scope of UK A-Level Chemistry courses).
The reason for this description of acid anhydrides as the theoretical result of dehydration of carboxylic acids is to explain and so make it easier to remember that acid anhydrides may be called acids because they may (in some cases) be produced from carboxylic acids, and that they may be called anhydrides because because they may (in some cases) be produced by removing water (from acids): The word 'anhydride' means 'without water'.
Names and Structures of simple Acid Anhydrides
A series of linear acid anhydrides is shown below with the molecular structure of each acid anhydride in the series drawn out in full (showing bond types but not accurate bond angles or, therefore, realistic molecular shapes).
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Examples of other names (synonyns) |
|
1 |
formic anhydride |
 |
|
2 |
ethanoic anhydride |
 |
|
3 |
propanoic anhydride |
 |
|
4 |
butanoic anhydride |
 |
|
5 |
pentanoic anhydride |
 |
|
6 |
hexanoic anhydride |
 |
|
7 |
heptanoic anhydride |
 |
|
8 |
octanoic anhydride |
 |
|
9 |
nonanoic anhydride |
 |
|
10 |
decanoic anhydride |
|
|
- Methanoic anhydride (C2H2O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Ethanoic anhydride (C4H6O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Propanoic anhydride (C6H10O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Butanoic anhydride (C8H14O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Pentanoic anhydride (C10H18O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
- Hexanoic anhydride (C12H22O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Heptanoic anhydride (C14H26O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Octanoic anhydride (C16H30O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Nonanoic anhydride (C18H34O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - Decanoic anhydride (C20H38O3)
Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:









